LANXESS
New “Lustran” ABS grade for medical use
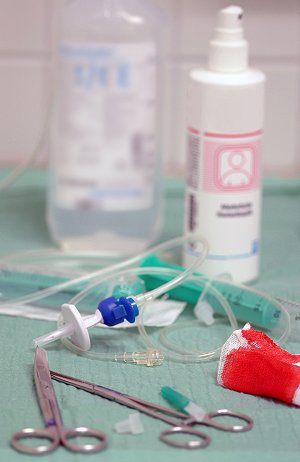 Lustran M205FC is ideal for components that require particular dimensionally stability including roller clamps and piercing pins. (Photo: Lanxess) |
A new ABS grade for the medical technology industry has been introduced by the Lustran Polymers business unit of Lanxess (Leverkusen / Germany; www.lanxess.com). “Lustran M205FC” is claimed to offer greater stiffness and strength than “Lustran M203FC” which has become established in the market.
Kirsten Wiele, Lustran’s medical technology industry manager says that the new grade is ideally suited to components that require particular dimensionally stability – including roller clamps and piercing pins. It also offers improved resistance to chemicals and has been identified for applications including components for inhalers, infusion sets and surgical instruments.
Kirsten Wiele, Lustran’s medical technology industry manager says that the new grade is ideally suited to components that require particular dimensionally stability – including roller clamps and piercing pins. It also offers improved resistance to chemicals and has been identified for applications including components for inhalers, infusion sets and surgical instruments.
10.07.2007 Plasteurope.com [208444]
Published on 10.07.2007
