AMCOR
Child-resistant closure for ophthalmic packaging / Development in response to CPSC regulations
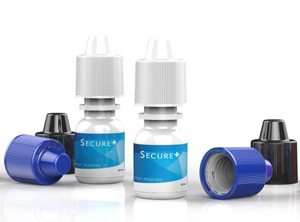 The two-piece child-resistant closure is injection moulded (Photo: Amcor) |
Packaging specialist Amcor Rigid Plastics (Abbotsford / Australia; www.amcor.com) has introduced a child-resistant closure for ophthalmic packaging. “SecurePlus” is a two-piece closure developed in response to US federal government regulations that require child-resistant closures for certain drug packaging.
“SecurePlus” is a 15-mm-diameter PP closure that uses a push-and-turn system that can be opened by adults. It is used for squeezable LLDPE eye drop containers ranging from 5 ml to 60 ml and replaces existing single-piece PP closures which are non-child resistant.
The injection moulded closure was developed in response to a ruling from the US Consumer Product Safety Commission (CPSC; www.cpsc.gov) that requires child-resistant packaging for any over-the-counter or prescription product containing the equivalent of 0.08 mg or more of imidazoline, a class of drugs that includes tetrahydrozoline, naphazoline, oxymetazoline and xylometazoline, in a single package.
“SecurePlus” is a 15-mm-diameter PP closure that uses a push-and-turn system that can be opened by adults. It is used for squeezable LLDPE eye drop containers ranging from 5 ml to 60 ml and replaces existing single-piece PP closures which are non-child resistant.
The injection moulded closure was developed in response to a ruling from the US Consumer Product Safety Commission (CPSC; www.cpsc.gov) that requires child-resistant packaging for any over-the-counter or prescription product containing the equivalent of 0.08 mg or more of imidazoline, a class of drugs that includes tetrahydrozoline, naphazoline, oxymetazoline and xylometazoline, in a single package.
02.11.2015 Plasteurope.com [232527-0]
Published on 02.11.2015
